Which Drugs Must Be Injected with a Light-proof Infusion Set?
- Share
- Issue Time
- Dec 22,2021
Summary
This article mainly introduces the medicines that need to be infused with a light-proof infusion set to prevent medical staff from operating errors in future use and causing medical accidents.
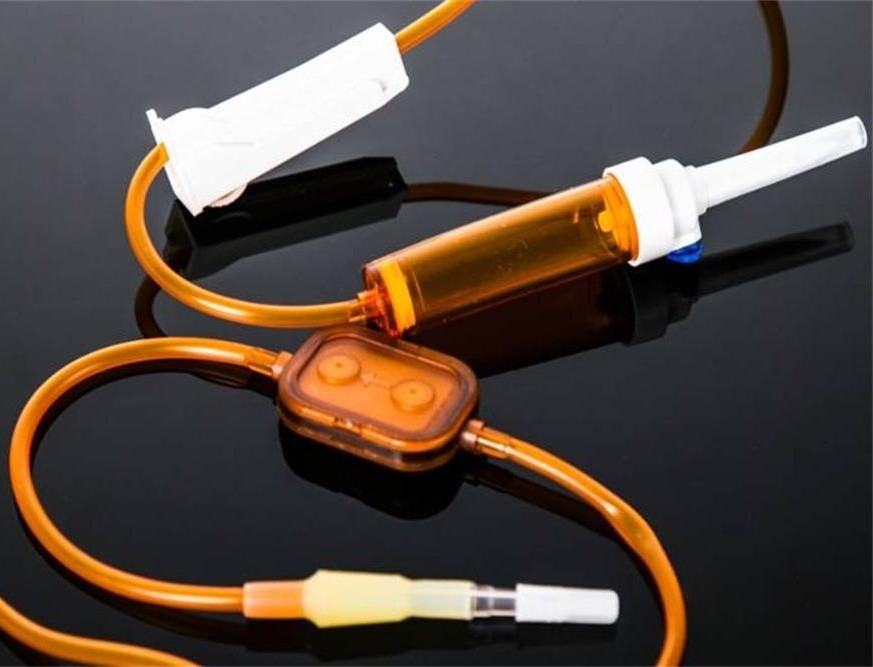
Some drugs have poor stability and are prone to oxidation, decomposition, discoloration, and other reactions when exposed to light. Especially for some chemotherapeutic drugs, complex reactions such as ring cleavage, rearrangement, hydrolysis, polymerization, oxidation, and isomerization can occur after dissolution. Light conditions can catalyze the progress of the above reaction. Therefore, care should be taken to avoid light during the production, transportation, storage, and installation of such drugs. Therefore, when we encounter the following drugs, we must use a light-proof infusion device for infusion.
Super light-proof medicine
Sodium nitroprusside is sensitive to light, and the solution stability is poor. The installation solution should be freshly prepared and protected from light. If it becomes dark brown, orange, or blue, it is prohibited. During intravenous drip, the infusion set should be wrapped with aluminum foil or opaque material to protect it from light.
First-level light-proof drugs
Levofloxacin hydrochloride, amphotericin B, doxorubicin. Levofloxacin hydrochloride can cause rare phototoxic reactions (incidence rate <0.1%). Excessive sun exposure and artificial ultraviolet light should be avoided when receiving this product. If photosensitivity or skin damage occurs, this product should be stopped, so during the infusion process should pay attention to avoid light; amphotericin B must be temporarily prepared during instillation, and infusion must be protected from light; adriamycin must be temporarily prepared during infusion, and infusion must be protected from light.
Secondary light protection drugs
Nimodipine, promethazine hydrochloride, chlorpromazine hydrochloride, water-soluble vitamins, epinephrine, isoproterenol, norepinephrine, dopamine, morphine, carboplatin, cisplatin, oxaliplatin, cyclophosphamide, cytarabine, methotrexate, fluorouracil.
As the active ingredient of nimodipine infusion is light sensitive, black, brown, or red glass syringes or infusion tubes should be used during infusion, or the infusion pump and infusion tube should be wrapped with light-impermeable materials or follow the doctor's advice. Promethazine hydrochloride and chlorpromazine hydrochloride are extremely easy to oxidize and change color under the action of light, metal ions, and oxygen.
Be careful to avoid light during intravenous drip. One-time dispensing should not be used for too long to ensure the effectiveness and safety of the medication. When water-soluble vitamins are added to glucose injection for infusion, they should be protected from light.
Epinephrine, isoproterenol, norepinephrine, dopamine, and morphine are easily oxidized and deteriorate under the influence of oxygen, metal ions, light, temperature, etc., suggesting that they should be protected from light during clinical use. When carboplatin, cisplatin, oxaliplatin, and cyclophosphamide are exposed to light, cisplatin injection will undergo photohydration reaction and photoredox reaction until metallic platinum is precipitated. Keep away from light when instilling.
After cytarabine, methotrexate, fluorouracil, etc. are dissolved and diluted, the aqueous solution is unstable, and the reaction is accelerated when exposed to light. The clinical installation should be protected from light.
Three-level light-proof drugs
Fat-soluble vitamins, mecobalamin, hydrocortisone, prednisone, furosemide, reserpine, procaine hydrochloride, pantoprazole sodium, etoposide, docetaxel, ondansetron, nitroglycerin, fat milk, medium/long-chain fat milk.
Etoposide, docetaxel, ondansetron, etc. have a certain effect on the stability of the drug under light irradiation, so light should be avoided as much as possible during the installation. Nitroglycerin, protect from light when using this product intravenously. Data such as fat emulsion, medium/long-chain fat emulsion, etc. show that in phototherapy when the fat emulsion is input at the same time, the lipid peroxide caused by light cannot be completely eliminated.
Therefore, as a preventive measure, it is recommended that during the phototherapy of newborns, the fat emulsion should be protected from light. For secondary and tertiary light-proof medicines, if the medicine is not dispensed, it should be kept away from light; if the medicine liquid changes color or deepens, it should be prohibited.
Other drugs that need to be protected from light
Cyclophosphamide, Cytarabine, Nimustine, Methotrexate, Fluorouracil, Doxorubicin, Mitoxantrone, Mitomycin, Epirubicin, Vincristine, Homoharringtonine, Anti-tumor drugs such as hydroxycamptothecin, paclitaxel, and dacarbazine are sensitive to light and heat and are unstable under sunlight, high temperature, and high humidity.
Vinorelbine, etoposide, topotecan, docetaxel, rituximab, ondansetron, arsenite, and other drugs are also causing certain influences under light exposure or high-temperature conditions.
The light-proof infusion set manufactured by BQ+ uses light-proof materials and precision filters, which not only meets the infusion needs of clinical drugs but also increase the infusion needs of drugs.
As a medical product manufacturer with extensive experience, BQ+ has developed and manufactured products that have passed various authoritative safety certifications and fully comply with strict medical requirements. We uphold a responsible attitude towards users and patients and control the quality of our products in an all-around way. Our products are also sold all over the world, if you want to buy our light-proof infusion set, please contact us immediately!