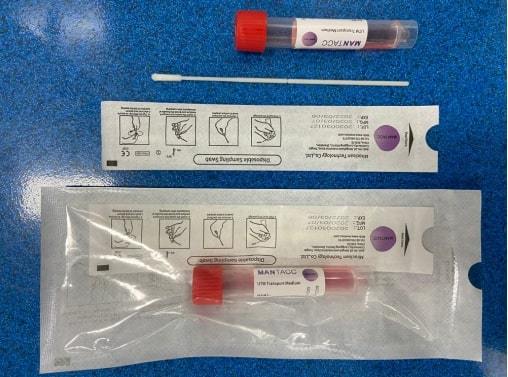
2019-nCoV Nucleic Acid Detection Kit
- Model
- PCR001
Item specifics
- Most popular market
- Germany,Australia, America,Asia
- Inventory
- Produce to order
- Lead time
- Check with sales Dept.
Review
Description
BQ PLUS offers a complete selection of New Coronavirus (2019-nCoV) Nucleic Acid Detection Kits
Product features:
1) 3 target detection: qualitative detection of ORF1ab gene, N gene and E gene, reduce recheck and improve detection efficiency
2) Full-process internal monitoring: internal control were used to effectively monitor the whole detection process of extraction, reverse transcription and amplification, to avoid false negative results
3) Anti-contamination system: UNG enzyme and dUTP were used to effectively prevent the contamination of PCR amplicon, to avoid false positive results
4) Rapid Turnaround Time: with rapid nucleic acid extractor and extraction reagent, the nucleic acid can be extracted and purified quickly and safely, and the detection of 96 samples can be completed in 2 hours
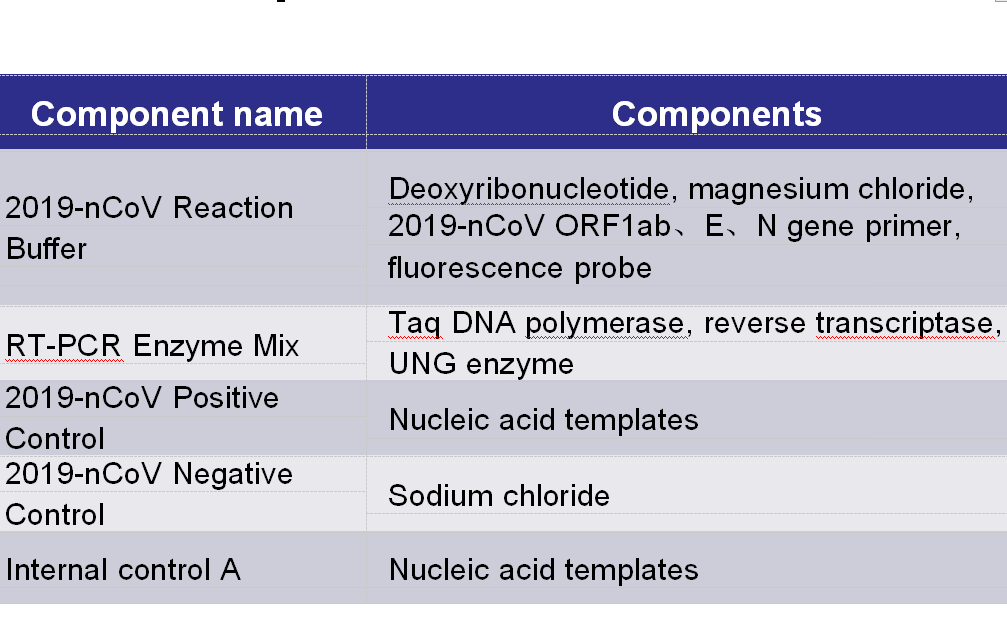
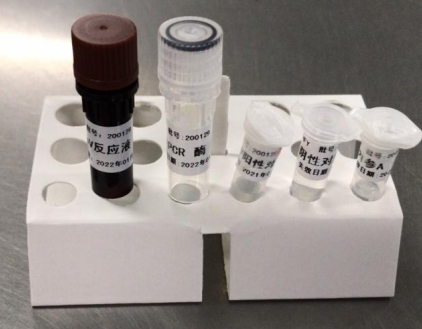
Main Components:
Note: the components in different batches of kits can not be interchanged, and the freeze-thaw times shall not exceed 5 times.
Detection Principle and Performance Evaluation:
1) Detection Principle:
– TaqMan real-time PCR technology, FAM channel detect ORF1ab gene, JOE channel detect N gene, ROX channel detect E gene, CY5 channel detect internal control
2) Performance Evaluation:
– Limit of detection: 300 copies/mL
– Precision: CV of CT value within / between batches is less than 5%
– Specificity: No cross reaction of other related pathogens with the same or similar infection site
Specification:
| Model | For Use With PCR Instruments | Certificate | Individual Packaging | Tests/kit | MOQ / lead time |
|---|---|---|---|---|---|
| PCR001 | ABI 7500, LightCycler480, SLAN96, LM2012, etc. with four-channel PCR instrument | ISO13485 CE | Very small box need dry-ice shipping | 48 | 10,000 tests 3-5 days |
Video
Other options available

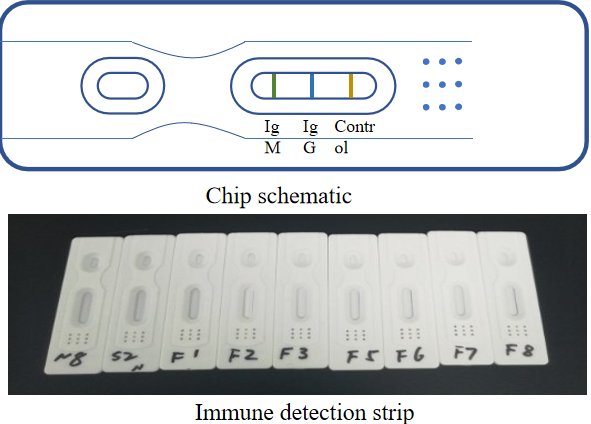
 IgM/IgG Antibody Fast Detection
IgM/IgG Antibody Fast Detection